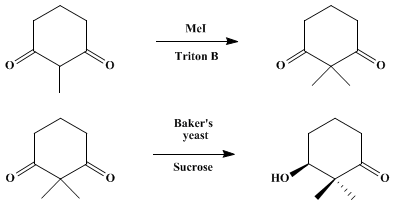
(S)-(+)-3-hydroxy-2,2-dimethylcyclohexanone
bp 85–87°C at 3.7 mm, [α]21D + 23.0° (CHCl3, c 2.0)
The spectral properties of (S)-(+)-3-hydroxy-2,2-dimethylcyclohexanone are as follows:
IR vmax (film) cm−1: 3470 (s), 1705 (s), 1120 (m), 1055 (s), 985 (s), 965 (m);
1H NMR (250 MHz, CDCl3) δ: 1.11 (s, 3 H), 1.15 (s, 3 H), 1.60–1.71 (m, 1 H), 1.76–1.86 (m, 1 H), 1.96–2.05 (m, 2 H), 2.16 (br s, 1 H), 2.35–2.45 (m, 2 H), 3.69 (dd, 1 H, J = 7.6, 2.9);
13C NMR (76 MHz, CDCl3) δ: 19.7, 20.7, 22.9, 29.0, 37.3, 51.3, 77.8, 215.3.
NOTE….Intermediate is
2,2-dimethylcyclohexane-1,3-dione bp 92–97°C (4 mm)
The spectra are as follows: 1H NMR (250 MHz, CDCl3) δ: 1.29 (s, 6 H), 1.93 (5 lines, 2 H, J = 6.5), 2.67 (t, 4 H, J = 6.9); 13C NMR (76 MHz, CDCl3) δ: 18.1, 22.3, 37.4, 61.8, 210.6.
- Department of Agricultural Chemistry, The University of Tokyo, Yayoi 1-1-1, Bunkyo-Ku, Tokyo 113, Japan.
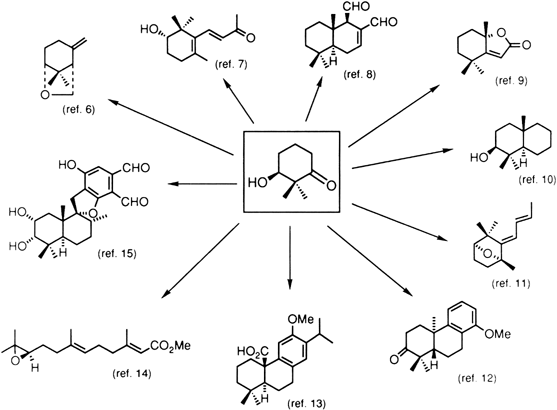
- Mekler, A. B.; Ramachandran, S.; Swaminathan, S.; Newman, M. S. Org. Synth., Coll. Vol. V 1973, 743, 3.
- Dale, J. A.; Mosher, H. S. J. Am. Chem. Soc. 1973, 95, 512.
- Kieslich, K. “Microbial Transformations of Non-Steroid Cyclic Compounds;” Georg Thieme; Stuttgart, 1976, pp. 28–31.
- Lu, Y.; Barth, G.; Kieslich, K.; Strong, P. D.; Duax, W. L.; Djerassi, C. J. Org. Chem. 1983, 48, 4549.
- Mori, K.; Mori, H. Tetrahedron 1985, 41, 5487.
- Yanai, M.; Sugai, T.; Mori, K. Agric. Biol. Chem. 1985, 49, 2373.
- Mori, K.; Watanabe, H. Tetrahedron 1986, 42, 273.
- Mori, K.; Nakazono, Y. Tetrahedron 1986, 42, 283.
- Mori, K.; Mori, H.; Yanai, M. Tetrahedron 1986, 42, 291.
- Mori, K.; Tamura, H. Tetrahedron 1986, 42, 2643.
- Sugai, T.; Tojo, H.; Mori, K. Agric. Biol. Chem. 1986, 50, 3127.
- Mori, K.; Mori, H. Tetrahedron 1986, 42, 5531.
- Mori, K.; Mori, H. Tetrahedron 1987, 43, 4097.
- Mori, K.; Komatsu, M. Liebigs Ann. Chem. 1988, 107.
Read all about Organic Spectroscopy on ORGANIC SPECTROSCOPY INTERNATIONAL 
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE














Sorry, the comment form is closed at this time.