One of the most ancient chemical operations carried out by humans is probably extracting aroma and flavor components from dried plant material. One of the most common examples is preparing a cup of tea or coffee. In the experiments described in this article, steam distillation is used for extracting oils from two popular spices – cinnamon bark and star anise. This is explained in Figure 1.
The main essential oils responsible for the aroma and flavour of these spices are trans-anethole [1-methoxy-4-(1-propenyl) benzene] from star anise and trans-cinnamaldehyde [(2E)-3-phenylprop-2-enal] from cinnamon bark.
Steam distillation is a method used for distilling immiscible liquids for which steam enables one of the immiscible phases. The two substances mix in the gaseous phase and co-distill however on cooling, the desired component separates from water since it is immiscible. Steam distillation is used for extracting perfume and to flavor oils from natural sources.
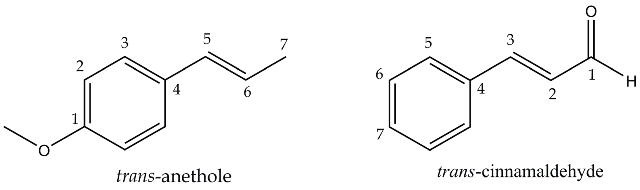
Figure 1. Essential oils to be extracted from spices: trans-anethole from star aniseand trans-cinnamaldehyde from cinnamon.
In order to extract the essential oil mainly comprising trans anethole from the star anise, a macrosccale direct technique for steam distillation is used. In this technique, steam is generated by heating water and the ground spice in the distillation flask as shown in Figure 2.
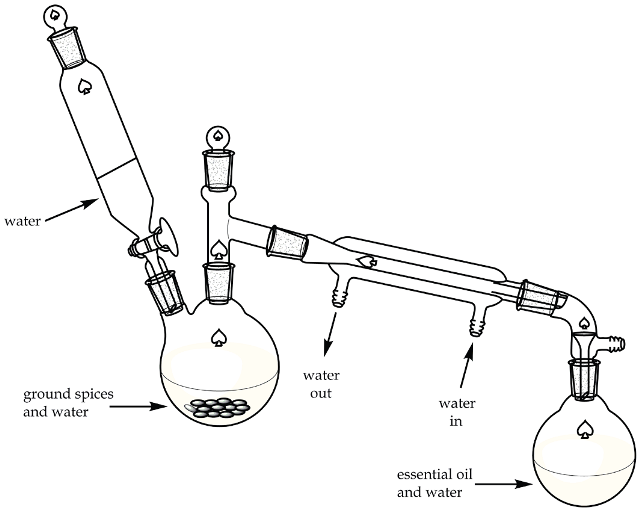
Figure 2. Direct method steam distillation apparatus.
Extraction of Trans-anethole from Star Anise
Procedure
Two whole star anise pods, around 3.5g is ground in a mortar and pestle as shown in Figure 3 and the ground material is kept in a 100mL 2-neck round bottom flask. Water is then added and distillation begins. After around 20mL of cloudy distillate is collected around 20mL water is added into the flask from the dropping funnel. Another 20mL of distillate is collected and then distillation is halted. The distillates are combined in a separatory funnel and extracted with diethyl ether (2 x 20mL). The ethereal layer is dried with anhydrous magnesium sulfate and the solution is filtered. The ether is removed with a rotary evaporator and the colourless star anise essential oil is collected. The yield is recorded.

Figure 3. Ground whole star anise seed pods.
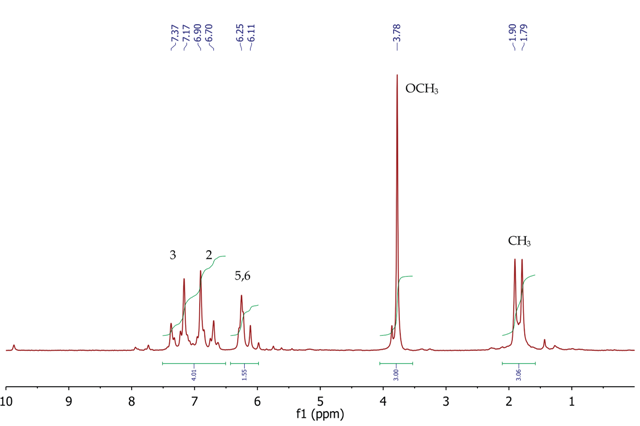
Figure 4. 1H NMR spectrum of trans-anethole, CDCl3
Figure 4 shows the 1H NMR spectrum of trans-anethole with a singlet (3H) at 3.78ppm, corresponding to the methoxy (OCH3) group. At 1.85ppm, a doublet (3H) is observed at 1.85 ppm for the methyl group at position 7. The two CH protons at fifth and sixth position appear between 6.11-6.25 ppm as a broad multiplet. The four aromatic protons at positions 2 and 3 resonate as a second-order AA’BB’ system with two multiplets centred at 6.80 and 7.27ppm. The COSY spectrum of trans anethole in Figure 5 and the
The COSY spectrum of trans-anethole (Figure 5) clearly shows correlations between two alkene protons at positions 5 and 6 and the methyl group at Position 7. COSY correlations are observed between four aromatic protons at positions 2 and 3.
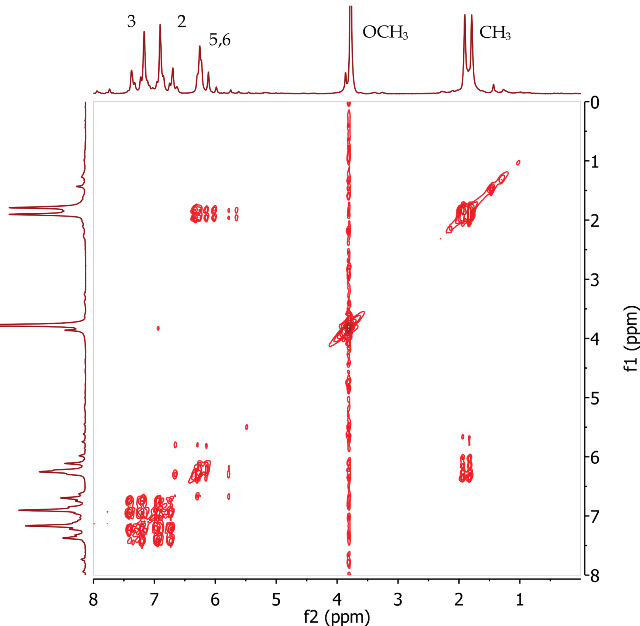
Figure 5. COSY spectrum of trans-anethole, CDCI3
Extraction of Trans-cinnamaldehyde from Cinnamon
A macroscale direct method for steam distillation is also used to extract the essential oil, which is composed primarily of trans-cinnamaldehyde, from the cinnamon spice. In this method, the steam is generated in situ by heating the ground dry spice material and water in the distillation flask. (figure 2)
Procedure
Grind two whole cinnamon sticks, around 5.5g in a mortar and pestle as shown in Figure 6, and place the ground material into a 100mL 2-neck round bottom flask. Around 40mL water is added and the distillation begins. After collecting 20mL of cloudy distillate more water is added to the distillation flask from the dropping funnel. Another 20mL of distillate is added and then the distillation is paused. The distillates are combined in a separatory funnel and extracted with diethyl ether (2x20 mL). The ether layer is dried with anhydrous magnesium sulfate and the solution is filtered. The ether is removed using a rotary evaporator and the colourless cinnamon essential oil is collected. The yield is recorded.

Figure 6. Ground sticks of cinnamon.
The 1H NMR spectrum of trans-cinnamaldehyde (Figure 7) shows a doublet (7.3Hz) at 9.73ppm for the aldehyde proton (CHO). The CH proton at position 2 can be seen as a doublet of doublets (16.0Hz, 7.3Hz) at 6.69ppm, as it is coupling to both the aldehyde proton (7.3Hz) and the other alkene CH proton at position 3. The 16.0Hz coupling constant between positions 2 and 3 shows the (E)- geometry about the double bond.
The five aromatic protons at positions 5, 6 and 7 can be seen as a broad multiplet centred at approximately 7.49 ppm. The CH proton at position 3 must resonate as a doublet as it is coupling to the CH proton at position 3, but its signal is overlapping with the signal for the aromatic protons.
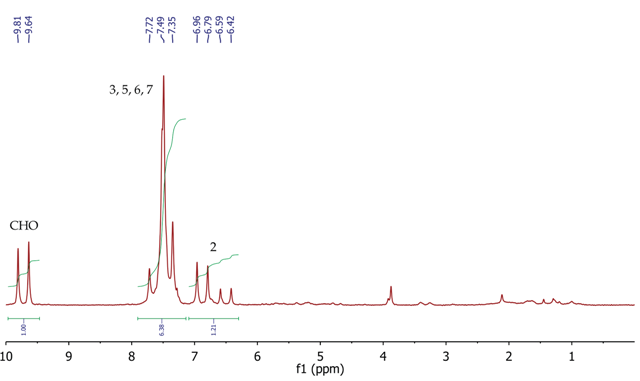
Figure 7. 1H NMR spectrum of trans-cinnamaidehyde, CDCI3.
The correlations between the aldehyde proton (CHO) at 9.73 ppm and the alkene CH proton at position 2 are seen in the COSY spectrum of trans-cinnamaldehyde (Figure 8). The CH protons at positions 2 and 3 are also coupling to each other. COSY correlations are also observed between the aromatic protons at positions 5, 6 and 7.
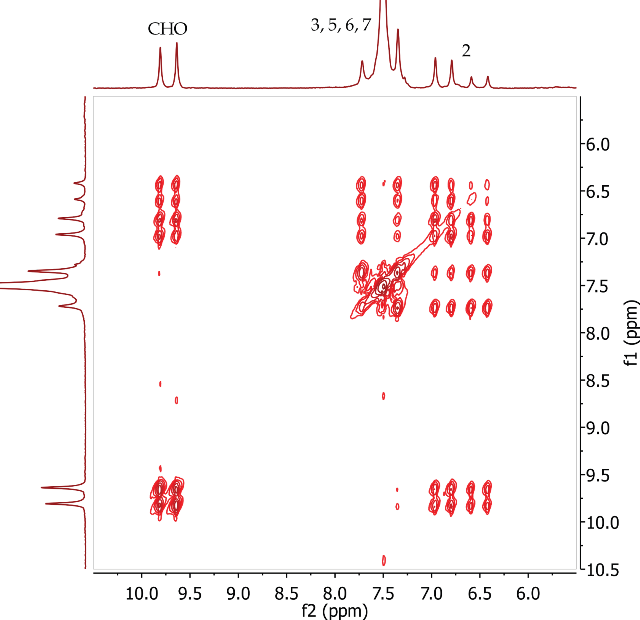
Figure 8. COSY spectrum of trans-cinnamaldehyde, CDCl3.
This information has been sourced, reviewed and adapted from materials provided by Magritek.
For more information on this source, please visit Magritek.