A new species of Cretalamna sensu stricto (Lamniformes, Otodontidae) from the Late Cretaceous (Santonian-Campanian) of Alabama, USA
- Published
- Accepted
- Received
- Academic Editor
- Fabien Knoll
- Subject Areas
- Biodiversity, Paleontology, Taxonomy
- Keywords
- Chondrichthyes, Elasmobranchii, Mooreville Chalk, Tombigbee Sand Member, Lamniformes
- Copyright
- © 2018 Ebersole and Ehret
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2018. A new species of Cretalamna sensu stricto (Lamniformes, Otodontidae) from the Late Cretaceous (Santonian-Campanian) of Alabama, USA. PeerJ 6:e4229 https://doi.org/10.7717/peerj.4229
Abstract
Decades of collecting from exposures of the Upper Cretaceous Tombigbee Sand Member of the Eutaw Formation and Mooreville Chalk in Alabama, USA has produced large numbers of isolated Cretalamna (sensu stricto) teeth. Many of these teeth had formerly been assigned to the extinct Late Cretaceous shark Cretalamna appendiculata (Agassiz, 1843), a taxon that is now considered largely restricted to the Turonian of Europe. Recent studies have shed light on the diversity of Late Cretaceous Cretalamna (s.s.) taxa, and here we recognize a new species from Alabama, Cretalamna bryanti. The teeth of C. bryanti sp. nov. appear aligned with the members of the Cretalamna borealis species group, but can be distinguished from these other species by a combination of the following: anterior teeth with a more pronounced and triangular lingual root protuberance, broader triangular cusp, and a taller root relative to the height of the crown; anteriorly situated lateroposterior teeth have a distally inclined or hooked main cusp and more than one pair of lateral cusplets; and lateroposterior teeth have a strong distally hooked main cusp and a root that is largely symmetrical in basal view. At present, C. bryanti sp. nov. is stratigraphically confined to the Santonian/Campanian Dicarinella asymetrica Sigal, 1952 and Globotruncanita elevata Brotzen, 1934 Planktonic Foraminiferal Zones within the Tombigbee Sand Member of the Eutaw Formation and Mooreville Chalk, and teeth have been collected from only four counties in central and western Alabama. The recognition of C. bryanti sp. nov. in Alabama adds to our knowledge on the diversity and distribution of Late Cretaceous otodontids in the region.
Introduction
Cretalamna is an extinct genus of lamniform shark that has been described globally from deposits ranging from the Albian (Early Cretaceous) to the Ypresian (Early Eocene) (Cappetta, 2012; Siversson et al., 2015). Glickman (1958) originally designated Otodus appendiculatus Agassiz, 1843 the type species for Cretalamna, however Siverson (1999) and Siversson et al. (2015) have since noted issues with Agassiz’s (1843) original syntypes. Not only did Agassiz (1843) not designate a holotype, but the specimens he figured appeared to represent a heterogeneous mix of several different genera (Siversson et al., 2015).
The compilation of taxa that comprised Agassiz’s (1843) original syntypes subsequently resulted in a broad interpretation regarding the morphology of C. appendiculata, consequently leading it to become a ‘waste basket’ taxon. To help remedy this situation, Siverson (1999) designated one of Agassiz’s (1843) former syntypes (pl. 32, fig. 10) the lectotype for C. appendiculata (sensu stricto). Later, Siversson et al. (2015) lent clarity to the diversity of Late Cretaceous Cretalamna-like species when they provided an amended diagnosis for C. appendiculata (s.s), and identified numerous C. appendiculata-like taxa spanning from the Cenomanian through the Campanian. Based on similarities in tooth morphology, Siversson et al. (2015) established three species groups for all but two of these Cretalamna (s.s.) species, the Cretalamna appendiculata group, the Cretalamna borealis group, and the Cretalamna hattini group.
In Alabama, decades of collecting from exposures of the Upper Cretaceous Tombigbee Sand Member of the Eutaw Formation and Mooreville Chalk has produced large numbers of documented Cretalamna teeth (Applegate, 1970; Thurmond & Jones, 1981; Ikejiri et al., 2013; Ciampaglio et al., 2013). Historically, most of these isolated teeth were assigned to C. appendiculata (see Applegate, 1970; Thurmond & Jones, 1981; Russell, 1988; Ciampaglio et al., 2013; Ikejiri et al., 2013), a taxon that is now considered largely confined to the Turonian of Europe (Siversson et al., 2015). The present study is the result of a reexamination of these Cretalamna teeth and their comparison to the various C. appendiculata-like species described by Siversson et al. (2015). This comparison has culminated in the recognition of a new species of Cretalamna (s.s.) from the Late Cretaceous of Alabama.
| Catalog Number | Stratigraphic unit | Locality | Tooth group | CH (mm) | TH (mm) | CW (mm) | TW (mm) | CT (mm) | Date collected | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| ALMNH 1068 | Lower Mooreville Chalk | Site ADa-3 | Upper right lateroposterior | 14.1 | 16.9 | 8.4 | N/A | 4.3 | 6/28/1988 | Mesial root lobe and cusplets missing, apex of crown worn |
| ALMNH 1164 | Lower Mooreville Chalk | Site ADa-3 | Upper left anteriorly situated lateroposterior; large morphology | 12.8 | N/A | 11.8 | 23.3 | 4.9 | 6/20/1988 | Entire apex broken |
| ALMNH 1407 | Lower Mooreville Chalk | Site ADa-3 | Upper right anterior | 16.6 | 21.7 | 6.9 | 13.6 | 3.4 | 7/8/1988 | distal cusplet broken off |
| ALMNH 1682 | Lower Mooreville Chalk | Site ADa-3 | Lower right anterior | 19.6 | 24.8 | 8.5 | 14.2 | 4.6 | 7/8/1988 | Distal root lobe broken |
| ALMNH 1719 | Lower Mooreville Chalk | Site ADa-3 | Lower left anterior | 18.1 | 23.8 | 8.9 | 16 | 4.2 | 6/23/1988 | |
| ALMNH 3322 | Lower Mooreville Chalk | Site ADa-3 | Upper right anterior | 18.7 | 23.8 | 7.7 | 14.4 | 3.6 | 1993 | Paratype |
| ALMNH 3330 | Lower Mooreville Chalk | Site ADa-19 | Upper left anteriorly situated lateroposterior | 15.3 | 21.1 | 10.5 | 20.1 | 4.2 | 6/30/1990 | |
| ALMNH 3331 | Lower Mooreville Chalk | Site ADa-20 | Upper right lateroposterior | 11.4 | 16.9 | 9.1 | 18 | 3.5 | 7/6/1993 | |
| ALMNH 3566 | Lower Mooreville Chalk | Site ADa-20 | Upper right anterior | 21.5 | 26.6 | 9.6 | 16.4 | 5.1 | 6/20/1993 | |
| ALMNH 3935 | Lower Mooreville Chalk | Site AGr-5 | Upper right anterior | 25.2 | 33.8 | 11.3 | 20.5 | 6 | 6/22/1994 | Broken root lobe |
| ALMNH 4190 | Tombigbee Sand Member | Site AGr-43 | Upper left lateroposterior | 8.9 | 12.7 | 7.4 | 14.6 | 2.8 | 7/8/1994 | |
| ALMNH 4517 | Lower Mooreville Chalk | Site ADa-19 | Upper left lateroposterior | 11.5 | 15 | 7.8 | 15.4 | 3.5 | 7/10/1996 | |
| ALMNH 5195.1 | Lower unnamed member of the Mooreville Chalk | Site AGr-5 | Upper left anteriorly situated lateroposterior; large morphology | 19.6 | 24.5 | 13.4 | 18.4 | 5.6 | 6/8/2005 | Mesial and distal roots damaged, distal cusplet broken |
| ALMNH 5195.2 | Lower unnamed member of the Mooreville Chalk | Site AGr-5 | Lower left anteriorly situated lateroposterior; large morphology | N/A | N/A | 13.8 | N/A | 5.4 | 6/8/2005 | Most of root and distal cusplets missing |
| ALMNH 5360 | Lower unnamed member of the Mooreville Chalk | Site AGr-4 | Upper right lateroposterior | 10 | 14.7 | 7.6 | 15.7 | 3.3 | 6/15/2005 | Apex of crown missing |
| ALMNH 6306 | Lower Mooreville Chalk | Site ADa-3 | Lower left anteriorly situated lateroposterior | 16.2 | 20.8 | 9.1 | 18.5 | 4.8 | 2010 | Paratype |
| ALMNH 6728 | Lower Mooreville Chalk | AGr-Exp. 33 | Upper left anteriorly situated lateroposterior; large morphology | 17.3 | 22.5 | 11.5 | 22.8 | 4.8 | 6/18/2003 | Mesial and distal root lobes abraded |
| ALMNH 6760 | Lower Mooreville Chalk | AGr-Exp. 33 | Upper right lateroposterior | 9.7 | 12.7 | 7.1 | 15.1 | 3.1 | 6/27/2013 | |
| ALMNH 8668 | Lower Mooreville Chalk | Site ADa-3 | Upper left anteriorly situated lateroposterior; large morphology | 18.2 | 24.8 | 14.8 | 26.2 | 5 | 6/25/2014 | |
| ALMNH 9216 | Lower Mooreville Chalk | Site ADa-3 | Lower right anteriorly situated lateroposterior; large morphology | 15.8 | 22.8 | 11.8 | 23.4 | 4.7 | 9/13/2015 | Apex of crown missing |
| ALMNH 9348 | Mooreville Chalk | AGr-Exp. 35 | Upper right anterior | 17.6 | 22.6 | 9.3 | 18.5 | 4.1 | 6/16/2015 | |
| ALMNH 9724 | Mooreville Chalk | Site ADa-3 | Lower right anterior | 20.3 | 26.5 | 8.2 | 15.9 | 4.6 | 11/13/2016 | |
| ALMNH 9878 | Mooreville Chalk | Site ADa-3 | Lower right anterior | 13.6 | 16.8 | 7.4 | 13.6 | 2.8 | 2/4/2017 | |
| ALMNH 15245 | Lower Mooreville Chalk | Site ADa-3 | Upper right lateroposterior | 12.6 | 16.3 | 7.5 | 15 | 3 | 1993/1994 | distal cusplet chipped |
| ALMNH 15250 | Tombigbee Sand Member | Site AGr-43 | Upper right anterior | 15.9 | 20 | 9.7 | 16.5 | 3.8 | 10/4/2017 | |
| MSC 1139.9 | Tombigbee Sand Member | Site AHl-1 | Upper left anteriorly situated lateroposterior | 13.6 | 18.3 | 10.2 | 18.9 | 3.1 | 7/17/1980 | |
| MSC 2984.1 | Lower Mooreville Chalk | Site ADa-3 | Upper right lateroposterior | 12.4 | 18.8 | 9.3 | 17.8 | 3.9 | 7/1/1982 | Holotype |
| MSC 5698 | Lower Mooreville Chalk | Site AGr-18 | Upper left anteriorly situated lateroposterior | 15 | 21 | 10.5 | 20.6 | 3.9 | 3/14/1983 | |
| MSC 5768 | Lower Mooreville Chalk | Site ADa-3 | Upper left lateroposterior | 12.2 | 18 | 8.6 | 16.51 | 3.2 | 7/5/1982 | Distal root lobe broken |
| MSC 26121 | Lower unnamed member of the Mooreville Chalk | Site AGr-4 | Upper right 1st? anteriorly situated lateroposterior | 15.8 | 21.9 | 10.5 | 20.5 | 4.3 | 6/10/2011 | |
| MSC 34051 | Lower Mooreville Chalk | Site ADa-20 | Upper left anteriorly situated lateroposterior | 15.2 | 21.9 | 10.4 | 23.1 | 4.1 | 8/16/2008 | |
| MSC 37499 | Lower unnamed member of the Mooreville Chalk | Site AMg-1 | Upper left lateroposterior; large morphology | 12.8 | 18.6 | 10.9 | N/A | 3.8 | 11/21/1993 | Distal base of crown and distal root lobe missing |
| MSC 37711 | Lower Mooreville Chalk | Site ADa-3 | Upper left anteriorly situated lateroposterior; large morphology | 20.2 | 26.8 | 14.2 | 26.3 | 5.9 | 5/31/2008 |
Herein we describe this new morphology and diagnose it against the numerous species of Late Cretaceous and Paleogene Cretalamna-like taxa from Alabama and elsewhere. In addition, we provide discussions on the various degrees of heterodonty observed within its dentition, document its stratigraphic and geographic distributions in the state, and discuss its taxonomic significance.
Material and methods
The teeth described in this study (n = 33) were identified by the current authors within the collections of the ALMNH and MSC. The specimens were collected from nine different localities in Alabama (see Table 1), all of which are referenced in this study by standard Alabama Paleontological Locality Numbers. Lithologic and stratigraphic information for each locality, or reference(s) containing this data, is provided in Appendix 1. However due to legalities regarding site access and protection, precise locality information for every collection site is not provided herein. Rather, this data is on file at both the ALMNH and MSC and is fully available to qualified researchers upon request. The precise localities for three specimens in our sample are unknown. Records associated with these teeth indicate they were collected from Mooreville Chalk gully exposures within the vicinity of the town of Clinton in Greene County, Alabama, USA. However, due to the number of gully sites within the area, it cannot be known for certain which exposure the teeth were derived. As a result, these three specimens are listed with the non-standard locality designations of AGr-Exp. 33 or AGr-Exp. 35 (see Table 1 and Appendix 1).
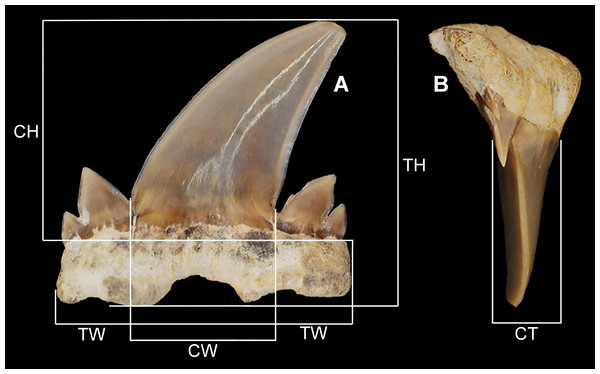
Figure 1: Diagram of tooth measurements taken as part of this study.
MSC 2984.1, holotype in (A) labial view and (B) mesial view. (CH) crown height. (CT) crown thickness. (CW) main cusp width. (TH) total height. (TW) total width.The examined specimens were collected from two types of localities, erosional gully exposures of Mooreville Chalk, and stream gravel bars containing vertebrate fossils derived from the Tombigbee Sand Member of the Eutaw Formation. The lone outlier is site AMg-1, a stream locality with exposures of both the Mooreville Chalk and Tombigbee Sand Member. Only one specimen (MSC 37499) was collected from this locality and it was derived from the Mooreville Chalk component exposed at this site. Most of the specimens (n = 26) were historically collected between the years 1980 and 2011. Because most of the examined specimens were not collected by the present authors, the direct methods of collection are not known for certain. However, after collecting at many of the same localities, the present authors can state with confidence that the specimens derived from localities consisting of erosional chalk gullies were obtained via surface collecting and those from stream localities were a result of either surface collection and/or screen washing matrix excavated from gravel bars.
Table 1 lists all the teeth examined as part of this study and includes the specimen repository, catalog number, locality number, stratigraphic unit, date collected, suggested tooth group, and tooth measurements. Standard measurements were taken of each tooth (Fig. 1), and notes on specimen breakage are reported when necessary. Because this new species is known only from isolated teeth, exact tooth positions within the jaw cannot be known for certain. Rather, the fossil teeth were placed within generalized tooth groups based on direct comparisons with extant jaw sets from various members of the Lamniformes (see Shimada, 2002). In particular, we examined jaw sets belonging to Isurus oxyrincus Rafinesque, 1810, Isurus paucus Guitart, 1966, and Lamna nasus (Bonnaterre, 1788) as these taxa have teeth with similar crown morphologies to those identified in this study. Teeth were placed into generalized anterior, anteriorly situated lateroposterior, and lateroposterior tooth groups based on a combination of distal crown inclination, number of lateral cusplets, and labial inclination of the roots (see the specific tooth group descriptions below for further discussions on these groupings). Commissural teeth are absent within our sample (likely due to a collecting bias), so teeth described herein from the lateroposterior files refer to those positioned within the inferred third lateroposterior hollow. Direct comparisons with extant jaw sets also assisted with the assigning of teeth to the upper or lower dentitions. On the extant jaw sets examined, teeth with a flat to lingually inclined main cusp were generally observed to occupy positions in the lower jaw (except for the first anterior tooth which has a weak lingual bend on the observed I. oxyrincus I. paucus, and L. nasus jaw sets). Teeth with a flat to labially inclined main cusp were generally observed to occupy the upper files.
The anatomical tooth terminology used in this study follows that of Shimada (2002), and tooth group terminology follows that of Siverson (1999). Higher taxonomic rankings used herein follow that of Nelson, Grande & Wilson (2016). Here we choose to follow Siversson et al. (2015) in the utilization of the spelling Cretalamna as opposed to Cretolamna (see Cappetta (2012) and Siversson et al. (2015) for competing arguments on the generic spelling). All specimens were photographed with a Nikon D80 Digital SLR camera with Tamron macro lens and rendered in Photoshop CC 2017 as part of the production of the presented figures. All specimens, including the holotype and paratype specimens, are housed in the scientific collections at either the ALMNH or MSC (see Table 1).
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new name contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: urn:lsid:zoobank.org:pub:F6C65E1D-19E6-4BA9-BEDD-DAFDB0BC27C9. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central and CLOCKSS.”
Geological Setting
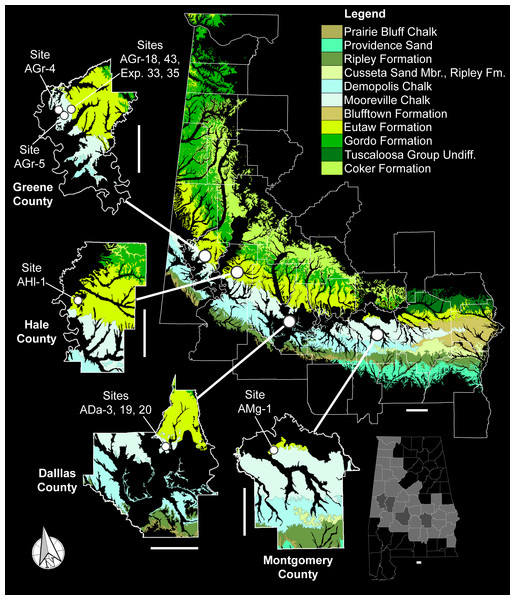
The teeth described in this study were collected over the course of 30 years from nine distinct localities located within Dallas, Greene, Hale, and Montgomery counties in Alabama, USA (Fig. 2). These four counties are located within the center and western parts of a region in Alabama known locally as the Black Belt (Ebersole & Dean, 2013). In Alabama, the Black Belt is a 440-km wide stretch of land that extends east to west across the center of the state and into the northwest corner (Adams et al., 1926), forming the southeastern edge of the Late Cretaceous Mississippi Embayment. Stratigraphically, the surface exposures within this region form a nearly complete sequence of Upper Cretaceous strata that ranges from the Cenomanian to nearly the end of the Maastrichtian (Mancini, Puckett & Tew, 1996; Puckett, 2005). The examined specimens were derived from two stratigraphic units within this region, the Tombigbee Sand Member of the Eutaw Formation and the Mooreville Chalk (Fig. 3).
Figure 2: Upper Cretaceous surface exposures in Alabama and Cretalamna bryanti sp. nov. specimen collecting localities.
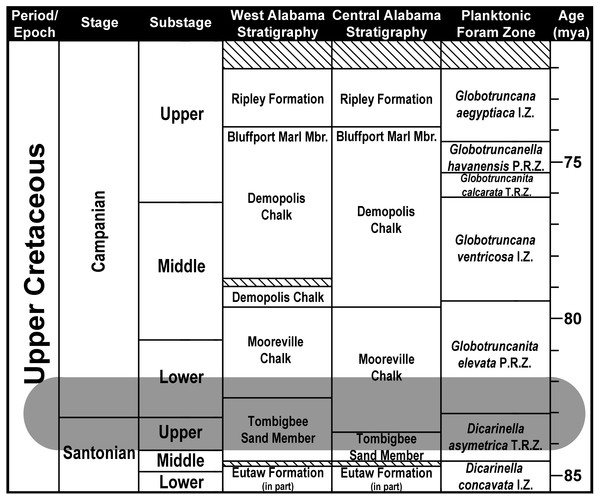
Scale bars equal 20 km.Figure 3: Santonian and Campanian surface stratigraphy in Alabama.
Modified from Mancini, Puckett & Tew (1996). Striped areas represent unconformities. Shaded area represents the stratigraphic range of Cretalamna bryanti sp. nov. in Alabama. Planktonic Foraminifera Zones after Caron (1985).The Tombigbee Sand is the uppermost member of the Eutaw Formation. In central and western Alabama, the Eutaw Formation uncomformably overlies the Turonian/Coniacian Gordo Formation, and underlies the Mooreville Chalk. The Mooreville Chalk is comprised of two members, a lower unnamed member and the upper Arcola Limestone (Raymond et al., 1988; Mancini, Puckett & Tew, 1996; Puckett, 2005). The Mooreville Chalk lies conformably above the Tombigbee Sand Member, and below the upper Campanian Demopolis Chalk (which in conjunction with the Mooreville Chalk makes up the lower half of the Selma Group). In central and western Alabama, the contact between the Tombigbee Sand Member and the Mooreville Chalk is time transgressive and straddles the Santonian/Campanian boundary. In central Alabama, this contact resides within the late Santonian, whereas in west Alabama the contact between these two units falls within early Campanian (Mancini, Puckett & Tew, 1996; Puckett, 2005; Prieto-Márquez, Erickson & Ebersole, 2016b).
The lithology of the Tombigbee Sand Member is varied (Mancini & Soens, 1994), but the unit is largely composed of highly glauconitic sand that may include clay, sandy chalk, or calcareous sandstone, and has abundant burrows and invertebrate and vertebrate remains (Raymond et al., 1988). The base of this unit is indicative of a high energy, tidally influenced nearshore environment, but the upper portion of the Tombigbee Sand Member represents a low energy marine shelf environment (Mancini & Soens, 1994). A large majority of the Mooreville Chalk is made up of a light gray fossiliferous chalk and chalky marl, however the lower few meters of the unit, an unnamed member, is composed of glauconitic and clayey marl (Raymond et al., 1988). The combination of these lithologies suggests the depositional environment of the Mooreville Chalk to be a calm, middle-shelf environment with dysoxic bottom conditions (Wylie & King, 1986; Mancini & Soens, 1994).
Figure 4: Mooreville Chalk gully exposures at the type section, site ADa-3 (Harrell Station), Dallas County, Alabama, USA.
Photograph courtesy of Dana J. Ehret.Although the examined specimens were collected from nine different localities, nearly half (15 of 33) were derived from the type locality, site ADa-3 in Dallas County, Alabama, USA (Fig. 2). Site ADa-3 is a 140-acre plot of land that is owned by the ALMNH and consists of extensive gully exposures of Mooreville Chalk, the only Late Cretaceous unit exposed at the site (Fig. 4). Studies by Puckett (2005) and Prieto-Márquez, Erickson & Ebersole (2016b) on the stratigraphy, ostracodes, planktonic foraminifera, and nannoflora at the site have shown that the Mooreville Chalk exposures at site ADa-3 fall within the Globotruncanita elevata Brotzen, 1934 Primary Foraminifera Range Zone (see Fig. 3), the upper half of the Acuminobrachycythere acuminata Hazel & Paulson, 1964 Ostracode Zone, and Nannofossil Zone CC-18a (Appendix 1). This places the exposures at this site within the Lower Mooreville Chalk with an age in the early, but not earliest, Campanian. Site ADa-3 is also the type locality for the hadrosauroid Lophorhothon atopus Langston, 1960. Detailed information on the other eight collecting localities can be found in Appendix 1.
Systematic Paleontology
| Class Chondrichthyes Huxley, 1880 |
| Subclass Euselachii Hay, 1902 |
| Infraclass Elasmobranchii Bonaparte, 1838 |
| Division Selachii Cope, 1871 |
| Superorder Galeomorphii Compagno, 1973 |
| Order Lamniformes Berg, 1958 |
| Family Otodontidae Glikman, 1964 |
| Genus Cretalamna Glikman, 1958 |
Type species
Otodus appendiculatus Agassiz, 1843, Upper Cretaceous (within the Cenomanian to early Coniacian interval), Lewes, England.
| Cretalamna bryanti Ebersole & Ehret sp. nov. |
| Figs. 1, 5–7 |
| LSID: urn:lsid:zoobank.org:act:1D850914-5CE2-4243-A0D9-0088B7046B13 |
| 2007 Serratolamna serrata (Agassiz, 1843); Shimada & Brereton, 2007: 106–110, fig. 2a–g. |
Etymology
bryanti = in honor of the Bryant family, whose commitment to education and ongoing support of the University of Alabama, the ALMNH, and MSC have enhanced the reputations and missions of all three institutions.
Holotype
MSC 2984.1, upper right lateroposterior tooth (Figs. 1, 7A–7E).
Type locality
Site ADa-3, Dallas County, Alabama, USA (Figs. 2 and 4). See Appendix 1 for additional locality information.
Type horizon
Lower Mooreville Chalk, early, but not earliest, Campanian, Globotruncanita elevata Brotzen, 1934 Primary Foraminifera Range Zone (see Fig. 3), upper half of the Acuminobrachycythere acuminata Hazel & Paulson, 1964 Ostracode Zone, Nannofossil Zone CC-18a (see Appendix 1).
Paratypes
ALMNH 3322, upper right anterior tooth (Figs. 5A–5E); ALMNH 6306, lower left anteriorly situated lateroposterior tooth (Figs. 6Z–6DD).
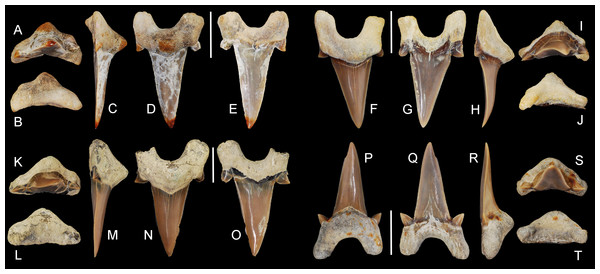
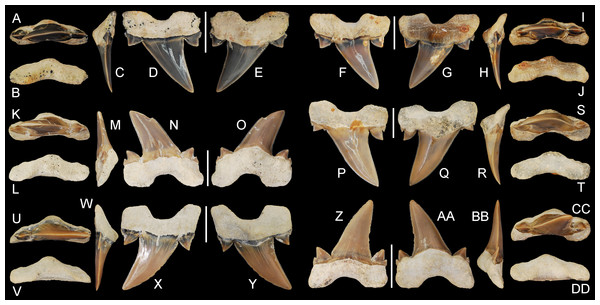
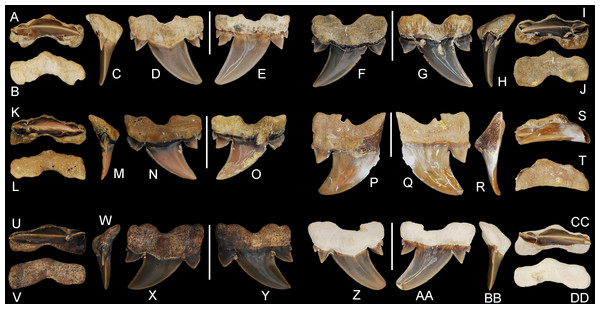
Figure 5: Cretalamna bryanti sp. nov. anterior teeth.
(A–E) ALMNH 3322, paratype, upper right anterior tooth in (A) oral, (B) basal, (C) mesial, (D) lingual, and (E) labial views. (F–J) ALMNH 3566, upper right anterior tooth in (F) lingual, (G) labial, (H) mesial, (I) oral, and (J) basal views. (K–O) ALMNH 3935, upper left anterior tooth, large morphology, in (K) oral, (L) basal, (M) mesial, (N) lingual, and (O) labial views. (P–T) ALMNH 9724, lower right anterior tooth in (P) lingual, (Q) labial, (R) mesial, (S) oral, and (T) basal views. Scale bars equal 1.0 cm.Figure 6: Cretalamna bryanti sp. nov. anteriorly situated lateroposterior teeth.
(A–E) ALMNH 3330, upper left anteriorly situated lateroposterior tooth in (A) oral, (B) basal, (C) mesial, (D) labial, and (E) lingual views. (F–J) MSC 34051, upper left anteriorly situated lateroposterior tooth in (F) labial, (G) lingual, (H) mesial, (I) oral, and (J) basal views. (K–O) ALMNH 9216, lower right anteriorly situated lateroposterior tooth, large morphology in (K) oral, (L) basal, (M) mesial, (N) labial, and (O) lingual views. (P–T) MSC 37711, upper left anteriorly situated lateroposterior tooth, large morphology, in (P) labial, (Q) lingual, (R) mesial, (S) oral, and (T) basal views. (U-Y) MSC 26121, upper right anteriorly situated lateroposterior tooth in (U) oral, (V) basal, (W) mesial, (X) labial, and (Y) lingual views. (Z–AA) ALMNH 6306, paratype, lower left anteriorly situated lateroposterior tooth, in (Z) labial, (AA) lingual, (BB) mesial, (CC) oral, and (DD) basal views. Scale bars equal 1.0 cm.Figure 7: Cretalamna bryanti sp. nov. posteriorly situated lateroposterior teeth.
(A–F) MSC 2984.1, holotype, upper right posteriorly situated lateroposterior tooth in (A) oral, (B) basal, (C) mesial, (D) lingual, and (E) labial views. (F–J) ALMNH 4517, upper left posteriorly situated lateroposterior tooth in (F) lingual, (G) labial, (H) mesial, (I) oral, and (J) basal views. (K–O) ALMNH 6760, upper right posteriorly situated lateroposterior tooth in (K) oral, (L) basal, (M) mesial, (N) lingual, and (O) labial views. (P–T) MSC 37499, upper left posteriorly situated lateroposterior tooth, large morphology, in (P) lingual, (Q) labial, (R) mesial, (S) oral, and (T) basal views. (U–Y) ALMNH 4190, upper left posteriorly situated lateroposterior tooth in (U) oral, (V) basal, (W) mesial, (X) lingual, and (Y) labial views. (Z–DD) ALMNH 5360, paratype, upper right posteriorly situated lateroposterior tooth in (Z) lingual, (AA) labial, (BB) mesial, (CC) oral, and (DD) basal views. Scale bars equal 1.0 cm.Additional Material
30 teeth: ALMNH 1068, ALMNH 1164, ALMNH 1407, ALMNH 1682, ALMNH 1719, ALMNH 3330 (Figs. 6A–6E), ALMNH 3331, ALMNH 3566 (Figs. 5F–5J), ALMNH 3935 (Figs. 5K–5O), ALMNH 4190 (Figs. 7U–7Y), ALMNH 4517 (Figs. 7F–7J), ALMNH 5195.1, ALMNH 5195.2, ALMNH 5360 (Figs. 7Z–7DD), ALMNH 6728, ALMNH 6760 (Figs. 7K–7O), ALMNH 8668, ALMNH 9216 (Figs. 6K–6O), ALMNH 9348, ALMNH 9724 (Figs. 5P–5T), ALMNH 9878, ALMNH 15245, ALMNH 15250, MSC 1139.9, MSC 5698, MSC 5768, MSC 26121 (Figs. 6U–6Y), MSC 34051 (Figs. 6F–6J), MSC 37499 (Figs. 7P–7T), MSC 37711 (Figs. 6P–6T). See Table 1 for detailed specimen information.
Diagnosis
Anterior teeth with a tall triangular main cusp, and single pair of divergent, lanceolate, lateral cusplets. Small secondary cusplets may be present on larger anterior teeth. Pronounced triangular root boss present with deep U-shaped basal concavity. Root lobes are long, range from rounded to angular (but are often angular distally), and are not as divergent as other C. appendiculata-like taxa. Anteriorly situated lateroposterior teeth have tall, triangular, distally inclined or distally hooked main cusp, and V-shaped neck (collar). One to two pairs of divergent, lanceolate, lateral cusplets present. Roots are symmetrical in basal view. Basal concavity is U-shaped. Lateroposterior teeth have a triangular main cusp with a strong distal hook, one to two pairs of divergent and lanceolate lateral cusplets, and labially inclined roots. Root lobes are scalloped on some teeth, and all have a shallow U-shaped basal concavity.
Description
Anterior teeth
Paratype specimen ALMNH 3322 (Figs. 5A–5E) best exemplifies the teeth in the anterior files. Identified as belonging to an upper right anterior position, this tooth measures 14.4 mm in greatest mesiodistal width and 23.8 mm in greatest apicobasal height (Table 1). The main cusp on the tooth is tall, triangular, and erect. In lingual and labial views, the distal edge of the main cusp is straight, while the upper two-thirds of the mesial edge is slightly convex. The basal half of the main cusp on the labial face is slightly convex and the apical half is flat. A shallow sulcus is present medially at the base of the main cusp on the labial face. The tooth has a single pair of strongly divergent cusplets. The cusplets have a slightly lanceolate outline in labial and lingual views. The labial and lingual faces of the cusplets are convex, but more so lingually. A shallow V-shaped neck (collar) is present at the lingual crown base. The mesial and distal cutting edges extend onto, and across, the lateral cusplets. The root lobes are rounded and divergent, with the mesial lobe being slightly more elongated than the distal lobe. The basal concavity is deep and U-shaped. A pronounced, triangular, lingual protuberance is present on the root. There is no nutritive groove, but several minute nutritive foramina are visible on the lingual root protuberance.
Overall, the main cusp on the anterior teeth tend to have a slightly increasing degree of distal inclination the closer they are positioned to the commissure (Fig. 5). Based on comparisons with a jaw set from the Recent Isurus paucus Guitart, 1966, both upper and lower anterior teeth are present within our sample. In profile view the upper anterior teeth can be distinguished by having a somewhat flat labial face on the main cusp and a labially bent apex (Figs. 5A–5O). In contrast, the labial face of the main cusp on the lower anterior teeth range from flat to having a slight lingual bend (Figs. 5P–5T). One tooth in our sample, ALMNH 3935 (Figs. 5K–5O), has a small secondary cusplet visible on the lateral edge of the medial cusplet. This specimen is the largest anterior tooth in our sample, measuring 33.8 mm in overall height (see Table 1), suggesting that the acquisition of secondary cusplets on the anterior teeth could be attributed to ontogeny.
Anteriorly situated lateroposterior teeth
Paratype specimen ALMNH 6306 (Figs. 6Z–6DD) best illustrates the anteriorly positioned lateroposterior teeth of C. bryanti sp. nov. This specimen measures 20.8 mm in total height and 18.5 mm in greatest mesiodistal width (Table 1). The main cusp on the tooth is tall and triangular. The mesial edge of the main cusp is slightly convex and the distal edge is concave, forming a distally inclined cusp. The labial and lingual faces of the main cusp are convex, but more so lingually. A pair of prominent lanceolate cusplets is present, and a minute secondary cusplet is visible on both the mesial and distal sides. This secondary cusplet appears under-developed and is largely united to the much larger medial cusplet. The primary cusplets are strongly divergent. The mesial and distal cutting edges extend to the base of the main cusp and across the lateral cusplets. The lingual crown face is smooth and a shallow V-shaped neck (collar) is present at the crown base. The labial crown face is also smooth. The root lobes are slightly divergent, labiolingually compressed, and the mesial root lobe is mesiodistally not as wide as the distal lobe, both of which are sub-angular. A deep U-shaped basal concavity is present, as is a lingual protuberance that is triangular in basal view. The root is nearly symmetrical in basal view. No nutritive groove is present but a series of minute nutritive foramina are located on the lingual root protuberance.
The anteriorly situated lateroposterior teeth appear to bear the characteristics of both the anterior and lateroposterior tooth morphologies. The presence of small secondary cusplets on many of these teeth appear to serve as a transition from the single-cuspleted anterior teeth (Fig. 5) to the often double-cuspleted lateroposterior teeth (Fig. 7). These teeth also have a distally inclined main cusp that is like those in the lateroposterior files, but differ by having a taller cusp (see Table 1) and by lacking a labially inclined root (compare profile views on Figs. 6–7). Although the labial crown face is smooth on most anteriorly situated lateroposterior teeth, faint enameloid folding is present at the base of the main cusp on some teeth (see Fig. 6X).
Several teeth in our sample lack secondary sets of lateral cusplets (see Figs. 6A–6E, 6U–6Y). On these teeth, the lateral edge on the primary set of cusplets is slightly extended laterally, likely homologous to a secondary cusplet. At times, a secondary cusplet is present mesially, but absent distally (Figs. 6F–6J). Upper teeth in our sample were differentiated by having a main cusp with a slight labial bend (Figs. 6A–6O, 6U–6Y). In contrast, the lower teeth have a cusp that ranges from flat to a having a slight lingual bend (Figs. 6Z–6DD).
Seven teeth in our sample (ALMNH 1164, ALMNH 5195.1-2, ALMNH 6728, ALMNH 8668, ALMNH 9216, and MSC 37711) are similar in overall morphology to those described above, but are larger in overall size and have a wider cusp base (Figs. 6K–6T, see Tab.1). The secondary set of lateral cusplets are often more defined on these larger teeth, and a minute denticle is at times present between the larger mesial cusplet and the base of the main cusp (Figs. 6P–6Q). Due to the large size of these teeth (up to 27 mm in height; see Table 1), we attribute these morphological differences to ontogeny and suggest that the cusp base gets wider and the secondary cusplets more defined as the shark reaches its maximum size.
Lateroposterior teeth
The teeth described below, referred herein as lateroposterior teeth, likely occupy the middle third of the inferred lateroposterior hollow. Teeth within this tooth group are best illustrated by the holotype, MSC 2984.1 (Figs. 1, 7A–7E), which is assigned here to an upper right lateroposterior tooth. This tooth measures 17.6 mm in greatest mesiodistal width and 16.7 mm in greatest apicobasal height (Table 1). The main cusp on the tooth is tall and triangular and has a strong distal hook due to having a very convex mesial edge and weakly concave distal edge. The lingual face of the main cusp is smooth and convex, whereas the labial face has a slight convexity and a small number of indistinct enameloid folds is present at the base. Two pairs of divergent lateral cusplets are present, with the medial pair being more than twice the height of the lateral set. The smaller, lateral pair of cusplets are largely separated from the medial pair. The lateral cusplets are lanceolate and have slightly convex mesial and distal edges. The mesial and distal cutting edges extend continuously from the apex of the main cusp across both pairs of lateral cusplets. A shallow V-shaped neck (collar) is present at the lingual crown base. The root lobes are angular and the base of the lobes are scalloped. The basal face of the root is flat, and the root has a distinct labial bend in profile view (see Fig. 7). The root has a shallow U-shaped basal concavity and a pointed lingual protuberance that is well demarcated in basal view. There is no nutritive groove, but a series of small nutritive foramina are located on the lingual protuberance.
Overall, the main cusp on the lateroposterior teeth appear to become slightly more distally hooked the closer they are positioned to the commissure. Due to the similarity of the lateroposterior teeth in our sample, they are interpreted to belong to the upper dentition as all have a straight to labially bent main cusp in profile view. The number of lateral cusplets is variable and ranges from three to four (at times a secondary cusplet is visible on the distal side only). One tooth in our sample, MSC 37499 (Figs. 7P–7T), is unique by having both a mesiodistally wider cusp base (10.9 mm vs. 7.1 to 9.3 mm) and much broader distal root lobe (the mesial lobe may also be expanded, but is not preserved). Despite having a wider cusp base, the crown height of MSC 37499 (12.8 mm) falls within the range of the other lateroposterior teeth in our sample (8.9 to 14.1 mm, see Table 1). It is our interpretation that this tooth represents a large, mature morphology and suggests that the cusp base and root lobes on the teeth get wider as the shark approaches its maximum size.
Remarks
Shimada & Brereton (2007) referred six teeth from our sample, MSC 1615.1, MSC 2984.1 (Figs. 1, 7A–7E), MSC 5698, MSC 5706, MSC 5768, and ALMNH 1164 (listed as PV 1988.20.73.4), to the Late Cretaceous taxon Serratolamna serrata (Agassiz, 1843). The teeth of Cretalamna bryanti sp. nov., however, differ appreciably from the type specimens of S. serrata illustrated by Agassiz (1843, vol. 3, pl. 32: 27–28) by lacking an elongated distal root lobe (which is present on all S. serrata teeth, regardless of position), by having one to two pairs of mesial and distal cusplets (lateral teeth of S. serrata generally have three distal and two mesial cusplets), and by lacking a nutritive groove (which is prominent on all S. serrata teeth; see Arambourg, 1952, pl. 15, figs. 1–41; Welton & Farish, 1993, p. 112, fig. 1–6).
As part of a later study (i.e., Ikejiri et al., 2013), one of the present authors (JE) examined several teeth with the C. bryanti sp. nov. morphology but interpreted them as representing large lateral teeth belonging to Cretalamna appendiculata (Agassiz, 1843). However, Siversson et al.’s (2015) study of C. appendiculata-like taxa lent clarity to the morphology of C. appendiculata (s.s.) teeth, solidifying to the present authors that the Alabama teeth do not belong to this latter taxon. Not only can the teeth of C. bryanti sp. nov. grow to much larger sizes (up to 27 mm in overall height; see Table 1), but the teeth of C. appendiculata (s.s.) never have more than a single pair of lateral cusplets (see Siversson et al., 2015, fig. 2). In addition, the main cusps on the anterior teeth of C. bryanti sp. nov. are much taller, more triangular, and have less bi-convex mesial and distal edges. Finally, the main cusps on the lateroposterior teeth of C. bryanti sp. nov. are much more distally hooked than those on C. appendiculata (s.s.).
Siversson et al. (2015) described numerous Cretalamna-like taxa, all but two of which could be placed into the Cretalamna appendiculata, Cretalamna borealis, or Cretalamna hattini species groups. Of these, the teeth of Cretalamna bryanti sp. nov. appear best aligned with the C. borealis species group which includes Cretalamna borealis Priem, 1897, Cretalamna ewelli Siversson et al., 2015, and Cretalamna gertericorum Siversson et al., 2015. Characteristics shared with the Cretalamna borealis species group include: (1) the presence of slender and elongated anterior teeth; (2) moderately wide lateroposterior teeth (which are mesiodistally much wider in the C. appendiculata and C. hattini groups); (3) a pronounced and well demarcated lingual protuberance on the root (especially apparent in basal view); (4) convex labial and lingual faces on the main cusp; (5) the unusual degree of distal cusp inclination on lateroposterior teeth; (6) the divergent and lanceolate shape of the lateral cusplets; (7) the presence of two pairs of lateral cusplets on some teeth; (8) the presence of scalloped root lobes on some teeth; and (9) the roots on the lateroposterior teeth often have a symmetrical sub-rectangular outline (Fig. 6).
In a comparison with the other members of the C. borealis species group, the anterior teeth of C. bryanti sp. nov. differ in several respects. First, in basal view, the lingual root protuberance on the anterior teeth of C. bryanti sp. nov. is generally more triangular and pronounced (Fig. 5) than on C. borealis (Siversson et al., 2015, figs. 9–10), C. ewelli (Siversson et al., 2015, fig. 12) or C. gertericorum (Siversson et al., 2015, fig. 11). Second, in labial or lingual views, the root lobes are less divergent (Fig. 5) than those on C. borealis, C. ewelli, or C. gertericorum, and the root lobes are longer than those on C. borealis and C. ewelli. Third, the basal concavity is more U-shaped on the anterior teeth of C. bryanti sp. nov., while slightly more V-shaped on C. borealis, C. ewelli, or C. gertericorum (a product of having more divergent root lobes). The basal concavity is also deeper on the anterior teeth of C. bryanti sp. nov. than on those on C. borealis and C. ewelli. Other differential characteristics on the C. bryanti sp. nov. anterior teeth include having a wider and more triangular main cusp than on C. ewelli and C. gertericorum, and a distal root lobe that is more angular than on C. borealis. Furthermore, the anterior teeth of C. bryanti sp. nov. have wider lateral cusplets and a more V-shaped neck (collar) than on C. ewelli. Moreover, the anterior teeth of C. bryanti sp. nov. have a broader and more triangular cusp when compared to similar teeth of the same size. In addition, when compared to the other C. borealis group species, the height of the root is greater relative to the height of the crown. Finally, the upper anterior teeth of C. bryanti sp. nov. have the unique combination of a relatively straight cusp that is labially inclined. This combination of characteristics has only been observed on the probable upper 3rd anterior position of C. borealis and C. ewelli (Siversson et al., 2015, fig. 12E).
The lateroposterior teeth of C. bryanti sp. nov. (Fig. 7) can be separated from those of C. ewelli by having three to four lateral cusplets on nearly all the specimens examined (as opposed to two on C. ewelli), a wider main cusp that is more distally hooked and has a more convex mesial edge, root lobes that are generally more angular, and a lingual neck (collar) that is more V-shaped. The C. bryanti sp. nov. lateroposterior teeth differ from those of C. gertericorum by having a slightly taller crown, a more triangular chevron, and the presence of two pairs of lateral cusplets on nearly all the teeth examined. A secondary set of lateral cusplets is present on only one of the C. gertericorum teeth figured by Siversson et al. (2015, fig. 11E3), a possible commissural tooth, but it is larger and less defined than those observed on C. bryanti sp. nov. (although this latter observation may be due to wear). When compared to those of C. borealis, the lateroposterior teeth of C. bryanti sp. nov. differ by having a more pronounced and triangular root boss, a less rectangular root outline in basal view, a basal concavity that is more U-shaped, a more V-shaped neck (collar), and a taller, more distally hooked main cusp.
Unfortunately, Siversson et al. (2015) did not figure any anteriorly situated lateroposterior teeth for C. borealis, C. ewelli, or C. gertericorum, negating a direct comparison for all three of these taxa with those in our sample. However, a photograph obtained of a C. borealis tooth (WAM 13.5.22) from Sweden compares favorably with one tooth in our sample, MSC 26121 (Figs. 6U–6Y), as both are likely from the upper first anteriorly situated lateroposterior position. Specimen MSC 26121 differs from this C. borealis tooth by having a more distally inclined main cusp, more divergent lateral cusplets, and a more U-shaped basal concavity.
At least two additional undocumented species of Cretalamna (s.s.) have been observed by the lead author (JE) to be present within Late Cretaceous deposits in Alabama. Both of these taxa have a cusp that is much shorter than those on C. bryanti sp. nov., they have a higher root relative to the height of the crown, a main cusp that is less distally inclined in the lateroposterior positions, and the teeth never have more than a single pair of lateral cusplets. These two taxa likely belong to Siversson et al.’s (2015) C. appendiculata or C. hattini species groups.
Elsewhere in the literature, Lauginiger & Hartstein (1983, p. 31, pl. 2, figs. 11–14, text fig. 13) described and figured teeth from the middle Maastrichtian Mt. Laurel Formation of Delaware that have a similar morphology to those of C. bryanti sp. nov. Originally assigned by Lauginiger & Hartstein (1983, pl. 2, figs. 12–13) to the subspecies “Cretolamna appendiculata pachyrhiza” (Herman, 1977), these teeth were later synonymized with C. borealis by Siversson et al. (2015). The teeth figured by Lauginiger & Hartstein (1983) differ from those of C. bryanti sp. nov. by having root lobes that are more rounded and more divergent, creating a wider basal concavity (Lauginiger & Hartstein, 1983, pl. 2, figs. 11–14). Furthermore, the lateral cusplets on the anteriorly situated lateroposterior and lateroposterior teeth of C. bryanti sp. nov. are much more separated from the main cusp than those figured by Lauginiger & Hartstein (1983), and none of the figured lateroposterior teeth have a secondary pair of lateral cusplets.
Arambourg (1952, fig. 14) figured 24 teeth from the Maastrichtian (Cretaceous) and Danian (Paleocene) of Morocco, several of which (figs. 14.1–11) appear superficially similar to the lateroposterior teeth of C. bryanti sp. nov. by having a laterally hooked main cusp and two pairs of lateral cusplets. However, the lateroposterior teeth of C. bryanti sp. nov. differ by generally having a slightly more distally hooked main cusp, a more concave labial crown face, and roots lobes that are more labially inclined. In addition, the dentition of C. bryanti sp. nov. differs by having anteriorly situated lateroposterior teeth with a hooked or distally inclined main cusp, while those illustrated for C. arambourgi are erect (see Arambourg, 1952, figs. 16–18). The anterior teeth associated with C. arambourgi also differ by having a much more pronounced and demarcated lingual protuberance (see Arambourg, 1952, figs. 12–13), and a main cusp that is shorter, mesiodistally narrower, and more bi-convex than those of C. bryanti sp. nov. Subsequently, these teeth, which were originally referred to “Lamna appendiculata” by Arambourg (1952), were assigned to a new subspecies, “Cretolamna appendiculata arambourgi”, by Cappetta & Case (1975), and later elevated to species level (i.e., C. arambourgi) by Siversson et al. (2015). The similarity of this taxon to C. bryanti sp. nov., as well to the other members of the C. borealis group, suggests that C. arambourgi may also have affinities with this species group.
Stratigraphic and geographic distribution of Cretalamna bryanti sp. nov.
The specimens in our sample were all collected from two Upper Cretaceous stratigraphic units in Alabama, the Tombigbee Sand Member of the Eutaw Formation and the overlying Mooreville Chalk (Fig. 3). In Alabama, the contact between the Tombigbee Sand Member and the Mooreville Chalk is time transgressive and straddles the Santonian/Campanian boundary. In central Alabama this contact falls within the upper Santonian, but in the western part of the state, the contact resides within the lower Campanian (Mancini, Puckett & Tew, 1996; Puckett, 2005). One specimen in our sample, MSC 37499 (Figs. 7P–7T), was derived from the lower unnamed member of the Mooreville Chalk at site AMg-1 in Montgomery County (Fig. 2). Collected from central Alabama, this tooth represents the stratigraphically oldest C. bryanti sp. nov. specimen within our sample, with an age that falls within the upper Santonian Dicarinella asymetrica Planktonic Foraminiferal Zone (see Fig. 3; Puckett, 2005), the last occurrence of which defines the Santonian/Campanian boundary (Caron, 1985). The remainder of the specimens were derived from Tombigbee Sand Member and Mooreville Chalk exposures in Dallas, Greene, and Hale counties, all located in west-central or western Alabama. In these parts of the state, the exposures of the Tombigbee Sand Member and Mooreville Chalk both fall within the early Campanian Globotruncanita elevata Planktonic Foraminiferal Zone (see Fig. 3), providing a bracketed late Santonian to early Campanian age for C. bryanti sp. nov. in Alabama, with all the specimens being derived from the D. asymetrica and G. elevata Planktonic Foraminiferal Zones.
In east Alabama, the Tombigbee Sand Member and Mooreville Chalk grade into the Santonian/Campanian Blufftown Formation. Although the Blufftown Formation is stratigraphically equivalent to the Tombigbee Sand Member and Mooreville Chalk of central and west Alabama, no teeth with the C. bryanti sp. nov. morphology were reported within the only published chondrichthyan study conducted within this formation (see Case & Schwimmer, 1988). The present absence of C. bryanti sp. nov. from the Blufftown Formation is likely a result of historical under-sampling within this unit, leading to a paucity of specimens being reposited in scientific collections.
C. bryanti sp. nov. specimens are also conspicuously absent from the Santonian, non-Tombigbee Sand Member component of the Eutaw Formation in Alabama, as well as from the upper Campanian Demopolis Chalk and the Campanian/Maastrichtian Ripley Formation, and Maastrichtian Prairie Bluff Chalk, and Providence Sand. However, in Alabama, field collecting within the non-Tombigbee component of the Eutaw Formation and the Demopolis Chalk has yielded far fewer vertebrate remains than the Tombigbee Sand Member or Mooreville Chalk (see Russell, 1988; Ikejiri et al., 2013). Furthermore, all the Maastrichtian deposits in the state have traditionally been under-sampled. Thus, it is our conclusion that collecting and preservation biases likely play a role in the limiting the stratigraphic range of C. bryanti sp. nov. in the state.
The present authors are not aware of any teeth with the C. bryanti sp. nov. morphology to have been figured or described elsewhere in the literature. However, it is possible that the previous lack of recognition of this morphology is the result of the misidentification of isolated teeth and their subsequent referral to other similar taxa. Thus, it is our conclusion that the geographic range of this taxon is likely much more extensive than reported here and deposits bearing similar age chondrichthyan assemblages may yield additional specimens.
Discussion
The recognition of C. bryanti sp. nov. from Alabama adds to our knowledge on the diversity of the Late Cretaceous members of Cretalamna (s.s.) and the Otodontidae. Understanding the relationship of the Otodontidae and the Cretoxyrhinidae within the Lamniformes has been a problematic subject for decades due to misidentification of type specimens (for example, see above for a discussion on Agassiz’s Cretalamna appendiculata type series) and disagreements about the taxonomy of various genera including Carcharodon, Otodus, Cretalamna, and Cretoxyrhina. The latter disagreements stem mainly from the poor preservation of shark cartilage, leading to a fossil record for the Chondrichthyes that is composed largely of isolated teeth.
Historically, certain members of the Otodontidae (i.e., Otodus (Carcharocles) and Otodus (Megaselachus)) were placed within the genus Carcharodon within the Lamnidae, and Cretalamna was placed within the Cretoxyrhinidae along with Cretoxyrhina and other assorted taxa (Agassiz, 1843; Cappetta, 1987; Applegate & Espinosa-Arrubarrena, 1996). In 1964, Glickman proposed that the mega-toothed sharks should be removed from the Lamnidae and placed within their own family. Subsequently, the limited distribution of his book (which was published in Russian) and the radicalness of his idea initially led to the lack of widespread acceptance of his proposal. However, new discoveries within the past 20 years has led to the majority acceptance of Glikman’s (1964) assertion that the mega-toothed sharks (i.e., the Otodus lineage) were a separate lineage from the lamnids and should be placed within the Otodontidae (Zhelezko & Kozlov, 1999; Cappetta, 2012; Ehret et al., 2012; Siversson et al., 2015). The Cretoxyrhinidae has since been shown to be a polyphyletic ‘wastebasket’ taxon (Siversson et al., 2015), and Cretalamna (s.s.) is currently recognized as belonging within the Otodontidae, having closer affinities to Otodus than to Cretoxyrhina (Siverson, 1999; Underwood & Cumbaa, 2010; Siversson et al., 2015).
The appearance of Otodus obliquus (Agassiz, 1843) in the Danian (Early Paleocene) of Alabama (see Ehret & Ebersole, 2014), and its morphological similarity with some of the Late Cretaceous Cretalamna (s.s.) taxa, certainly suggests that one of the species groups reported by Siversson et al. (2015) gave rise to the Otodus lineage. However, due to the complexity of the Cretalamna (s.s.) species complex and the likelihood that numerous Late Cretaceous members are yet to be described, at present it is not possible to ascertain for certain from which lineage Otodus may have been derived. Hopefully future studies on Late Cretaceous Cretalamna (s.s.) diversity will shed light on these complex evolutionary relationships.
Conclusions
Siversson et al.’s (2015) study of Late Cretaceous Cretalamna-like taxa has led to the present recognition of a new species of Cretalamna (s.s.) from Alabama, Cretalamna bryanti sp. nov. Multiple characteristics align this new taxon with Siversson et al.’s (2015) Cretalamna borealis species group including: elongated anterior teeth; a pronounced and demarcated lingual root protuberance; convex labial cusp face; strong distal cusp inclination in upper lateroposterior files; up to two pairs of divergent, lanceolate lateral cusplets; scalloped roots lobes on some teeth; and roots on lateroposterior teeth that are largely symmetrical in basal view. The anterior teeth of C. bryanti sp. nov. differ from the other members of this group by having root lobes that are less divergent (forming a more U-shaped basal concavity); and an erect main cusp on upper anterior teeth that are also labially inclined. The anteriorly situated lateroposterior and lateroposterior teeth differ by the presence of three to four well-defined lateral cusplets on most teeth in combination with a laterally inclined or strong distally hooked main cusp.
Our sample of C. bryanti sp. nov. teeth indicate that the dentition of this shark had varying degrees of monognathic, disjunct, and dignathic heterodonty, and the presence of a few larger teeth with slight morphological differences suggests a degree of ontogenetic heterodonty was present as well. At this time, C. bryanti sp. nov. appears stratigraphically confined to the Santonian/Campanian Dicarinella asymetrica and Globotruncanita elevata Planktonic Foraminiferal Zones within the Tombigbee Sand Member of the Eutaw Formation and Mooreville Chalk in Alabama. The depositional settings for these units suggest C. bryanti sp. nov. favored low energy shallow, middle shelf, environments. In Alabama, this species is currently confined to four counties in the central and western parts of the state, but the absence of this taxon from other counties and stratigraphic units could be the result of collecting and preservation biases. The lack of recognition of this morphology elsewhere is likely the result of the misidentification of isolated teeth and their subsequent referral to morphologically similar taxa.
The recognition of C. bryanti sp. nov. from Alabama adds to our knowledge on the diversity and distribution of otodontids in North America. With the presence of several recently recognized species of Late Cretaceous Cretalamna (s.s.) in the United States (see Siversson et al., 2015), and recently described Paleogene otodontids from Alabama (Ehret & Ebersole, 2014), we are beginning to gain clarity on the diversity and distribution of otodontids in the region.